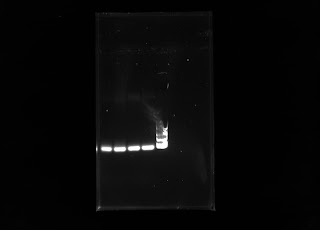
This week I ran another PCR with my DNA samples with the same chloroplast primers. The first PCR run included 10 microliters of distilled water, 10 microliters DNA samples, and 20 microliters of the plant master mix that included the chloroplast primers. The only changes to the protocol was increasing the amount of water to 15 microliters and changing the amount of DNA sample to 5 microliters. The amount of plant master mix into the PCR tube stayed the same. The idea was for the primers to have a higher chance of targeting the small amount of DNA to increase amplification. The result of this PCR after electrophoresis is presented below in this gel:
We assumed the ladder ran off a bit weird due to how the gels were made. I'll need to run another fresh gel with my PCR samples. For the mean time, I'll be working on amplifying the DNA samples with other primers in the lab that include primers 1-7.
Thursday, March 31, 2016
Thursday, March 24, 2016
Harvesting from Multiple Tusken Raiders & Amplification
Before spring break, I was able to collect spines from a population of teddy-bear cholla (cylindropuntia bigelovii) at the dreamy draw hike. Below are some pictures of the population of cholla cacti, and both of my mentors got in my way.
I ran a PCR on my five samples with chloroplast primers and the results were not what I expected. Since I did not know what kind of primers were in the master mix, I did not know which primer to avoid when running a PCR with the
DNA samples. The next step was to run a RAPD PCR with primers we have in the lab to see if the amplification would be better than the amplification from the Chloroplast primers. Below is a gel of the DNA samples amplified with the chloroplast primers.
Here is also a video of one of our top highlights at the Dreamy Draw hike:
Thursday, March 10, 2016
The Future of the Tusken Raider Spines
This week I was able to run a gel on five samples of DNA I have extracted. The most recent extraction was of prickly pear spines for which I changed the protocol by the time I conducted the extraction. The sample was cleaned in twine and bleach before it was heated for three hours at 70 degrees Celsius. I ran the sample along with four others, including the control, but I was not able to see any bands. The control sample of Trifolium sp. had a band in the first lane, but the rest of the cactus spine samples did not. The next step is to run a PCR and I hope to see DNA banding after the PCR samples undergo electrophoresis. Here is my gel below:
Thursday, March 3, 2016
Spines from a Tusken Raider's Head
This week I worked on extracting DNA from prickly pear spines in hopes of perfecting a protocol in order to see some banding in my gels. I was able to collect only 40 mg of cholla spines, so Josh was able to collect some prickly pear spines for me in his front yard. Below is a picture of how much 40 mg of cholla spines looks like. It is not very much and I need a packed tube of cholla spines to conduct multiple extractions of 150 mg each.
The difference in this protocol I conducted this week was how there was a 3-hour incubation period at seventy degrees Celsius. It seemed as though this worked because the spines looked like they had turned to a pulp and there would be a more likely chance of extracting DNA. I also used small scissors to cut up the pieces and did some grinding. After running the gel though, I did not see any DNA bands. The next suggestion, given to me by Matt, was to grind the spines with a pestle and mortar to create a powdered substance from the spines and use 150 mg of that plant material for my next extraction. I am hoping it will work.
Subscribe to:
Comments (Atom)